

Neuro-AI is a company built around delivering advanced neuroimaging capabilities to biopharmaceutical companies, clinical research organizations, marketing organizations, and patients worldwide. Our team consists of leading scientists, clinicians and technology experts.
Neuroimaging can play a role in drug development at all stages of the process, pre-clinical to phase IV. In pre-clinical it can be used for validating animal models. In phase I, it can be used to better establish dose response curves, aid in the shifting the proof of concept (POC) from late to early phase, and identifying additional/alternative targets for late stage study. In phases II & III, it can aid and/or serve, thanks to the recent passage of passage of the 21st Century Cures ActI, as proof of efficacy. In phase IV, it can be used to provide differentiation between available treatments.
Focusing here just on POC, the CNS drug pipeline has stymied, with CNS drugs 45% less likely to proceed to regulatory filling than non-CNS drugsII. Failures in CNS drugs are due in large part to failures to demonstrate efficacy in Phase III, with rates significantly higher than non-CNS drugsII. While phase III attrition rates are significantly higher for CNS drugs, it is not the case for phase II. Putting it in other words CNS drugs fail late in trials predominately for efficacy when compared to other drugs. These late stage failures are costly both in time and in dollars.
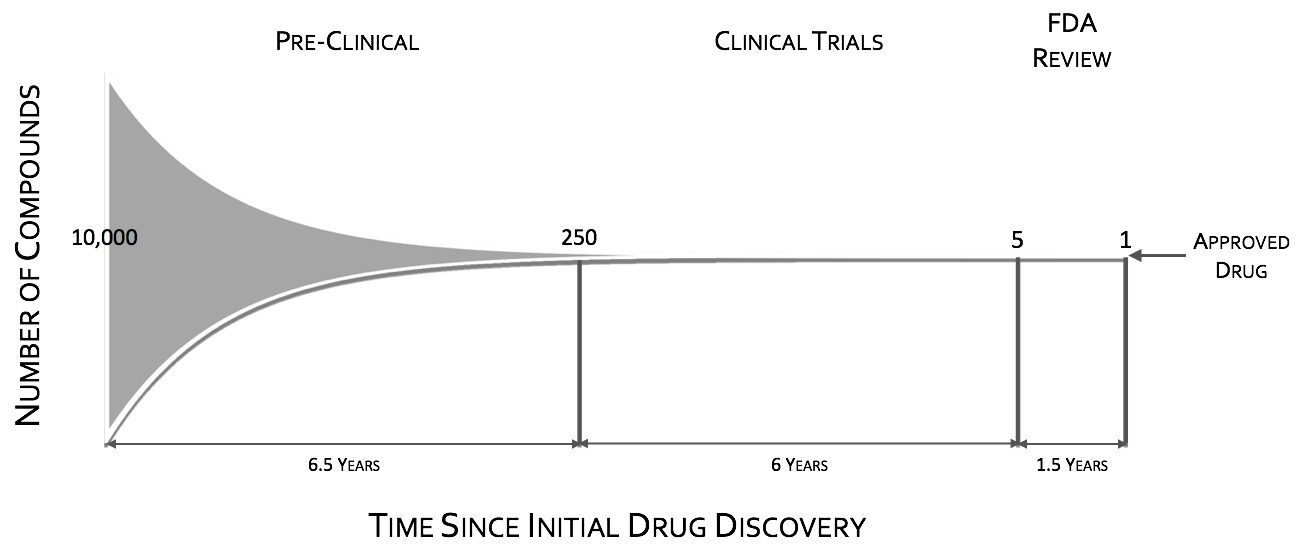

To quote the former director of National Institutes of Mental Health (NIMH), Dr. Thomas InselIII, “Given that over 95% of compounds fail during the clinical phases of development (a fact not appreciated by looking at the published literature which is biased towards positive results), success may require rapid failures in order to conserve resources by moving quickly to the next candidate (“fast-fail”IV)." The “fast-fail, quick win” model (see figure below) is one where the proof of concept (POC) is shifted earlier in the clinical trials, from phases II & III to phase I, from on or after the first effective dose (FED) to the first human dose (FHD) or shortly thereafter.
Shifting the POC earlier, will increase attrition in earlier stages, but will also increase the probability of success in the new molecular entities (NME’s) that make it through phase I. This “fast-fail” approach allows a culling of the pipeline early on, reducing costV. The savings estimate due to this shift is on the order of $30 Million dollars in out-of-pocket expenses per NMEIV. This savings can be used to fund approximately 2 additional NME phase I studiesIV.

This POC shift to early phase requires the support of surrogate measures. Positron Emission Tomography (PET) has long been considered the main avenue for surrogate makers in CNS clinical trials, whether through biodistribution, receptor occupancy/binding or measuring metabolic changes in the target using FDG or FDOPAVI. Unfortunately, biodistribution and receptor occupancy/binding are only a part of a POC, that is, they show that the drug in question is finding the target, but show nothing on whether or not it is modulating the target circuit in a meaningful way. FDG & FDOPA have the potential to measure target modulation, but they are slow (low temporal resolution) and blunt (low spatial resolution) tools, and as such they miss many meaningful signals. Where PET fails in measuring functional change other neuroimaging modalities can be brought to bear. These include but are not limited to: fMRI, ASL, qEEG, EEG-fMRI, EEG source imaging, NIRS, & MEG.
FMRI and other imaging modalities such as qEEG, EEG-fMRI, EEG source, MEG and NIRS are quantitative based tools, providing researchers and business leaders with statistical decision making power.
Since its inception roughly 25 years ago functional magnetic resonance imagining (fMRI) VII, VIII, & IX has opened up a new realm of possibilities with neuroimaging. It has confirmedX, debunkedXI as well as opened new understandingsXII of how the mind works. When taking the whole family of non-invasive neuroimaging modalities into account it has by far the highest spatial resolution with some studies using resolutions on the order of sub millimeterX. With all of its accolades and the beautiful pictures it creates, fMRI still has highly limited temporal resolution with the current upper limit of its sampling rate being on the order of hundreds of millisecondsXIII.
Electroencephalography (EEG) fMRI’s much older cousin has been around for over a centuryXIV and gave some of the first views into the active mind. Even now it is still the key measurement for sleep stagingXV, XVI and for diagnosing many neurological disorders such as epilepsyXVII and schizophreniaXVIII. EEG, whose instrumentation is technologically primitive when compared with that of fMRI, leaves it in the proverbial dust in that EEG has essentially limitless temporal resolution. EEG spatial resolution on the other hand is more of an inference than a certainty in that in its native domain it a measurement of electrical potential differences on the scalp. Transforming these measures across the scalp into the space of the brain is done through a process referred to as source localization. Source localization’s resolution is on the order of one to two centimetersXIX.
Our team and advisors have years of experience implementing advanced imaging solutions across a wide range of applications, including for global Clinical Research Organizations and major pharmaceutical companies.
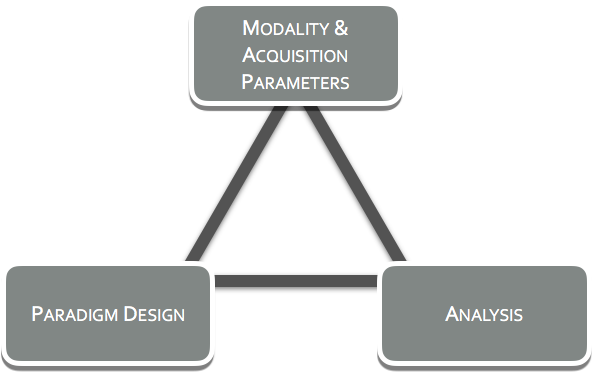
Too often neuroimaging is assumed to be a black box, where all one needs to do is insert a brain and its secrets shall be revealed. In truth, neuroimaging should never be treated this way, due to the fact that the choices on the acquisition modality, paradigm design, signal processing, statistics and data analysis all shape the output. How the choices affect the output is predictable and this is where Neuro-AI can help.
At Neuro-AI, we believe exploratory, the ‘let's-try-it and see’ approach has it merit and its role, but this is not at the stage of clinical trials. We believe asking targeted questions, with careful thought going into the selection of the modality(ies) to be used, the design of the paradigm(s), and to the analyses that will be performed on the acquired data. We at Neuro-AI call this the Neuroimaging Trifecta and it is important to establish in the advance of a study as the three play into one another.
Neuro-AI’s luminary team can speed up the transition of drugs from development into the real world, reducing both the time and cost associated with getting treatments into the hands of patients & caregivers that need them most. Our expertise in neuroimaging helps our pharmaceutical partners streamline early and late phase clinical trial drug testing programs, while unlocking new opportunities for existing and future therapeutics. We provide comprehensive expertise across imaging modalities from MRI, to NIRS to EEG as well as multimodal imaging solutions for all phases of clinical trials. Some of our service offerings include, but are not limited to:
Cameron Rodriguez, PhD, Founder

Cameron is an expert on the implementation of EEG and fMRI. He has developed the leading technologies in gradient artifact correction and balistocardiogram artifact timing. He has also developed techniques to simplify and increase the reliability of EEG source imaging. Beyond Neuro-AI, Cameron also co-founded Se Quepa, a company that develops novel and simple technologies to aid in neuroimaging. In addition, he teaches Principles of Neuroimaging at UCLA. Previously, he has served as Technical Director for New Health Partners LLC and as the Head of Technical Development at UCLA’s Staglin Center for Cognitive Neuroscience. Beyond MRI and EEG, he has a multidisciplinary background, including research in radio astronomy at the Arecibo Radio Telescope in Puerto Rico as well as developmental neurobiology and human mesenchymal stem cells.
Mark S. Cohen, PhD, Scientific Advisor

Mark's training is equal parts engineering and neuroscience. His contributions include his critical role in the development of practical echo-planar scanning, ultra-fast MRI applications, contrast-based and BOLD functional MRI, applications of linear systems analysis to increase fMRI sensitivity and resolution, and concurrent recordings of EEG and fMRI to better understand brain dynamics and distributed processing. He and his lab have contributed to an understanding of the power of pattern recognition and machine learning to both interpret/classify neural data and as a source of discovery of the processes that result in cognition, perception, emotion and pathology. Mark holds appointments in the UCLA Departments of Psychiatry, Neurology, Radiology, Biomedical Engineering, Psychology and Biomedical Physics and is a member of the California NanoSystems Institute.
Ariana Anderson, PhD, Statistical Advisor

Ariana is an Assistant Research Statistician at UCLA, an NIH funded investigator, and a recent recipient of the Burroughs Wellcome Fund 2014 Career Award at the Scientific Interface. She was a featured speaker at the Institute of Medicine on how to address the placebo response in drug studies and worked with Dr. Karl Friston at University College, London on approaches to relaxing Bayesian priors in Dynamic Causal Models. She previously served as an early career reviewer (ECR) for the NIH study section: Small Business: Neuro/Psychopathology, Lifespan Development, and STEM Education (RPHB 12) as well as a reviewer for the NSF.