

The CNS drug pipeline has stymied, with CNS drugs 45% less likely to proceed to regulatory filling than non-CNS drugsI. Failures in CNS drugs are due in large part to failures to demonstrate efficacy in Phase III, with rates significantly higher than non-CNS drugsI. While Phase III attrition rates are significantly higher for CNS drugs, it is not the case for Phase II. Putting it in other words CNS drugs fail late in trials predominately for efficacy when compared to other drugs. These late stage failures are costly both in time and in dollars.
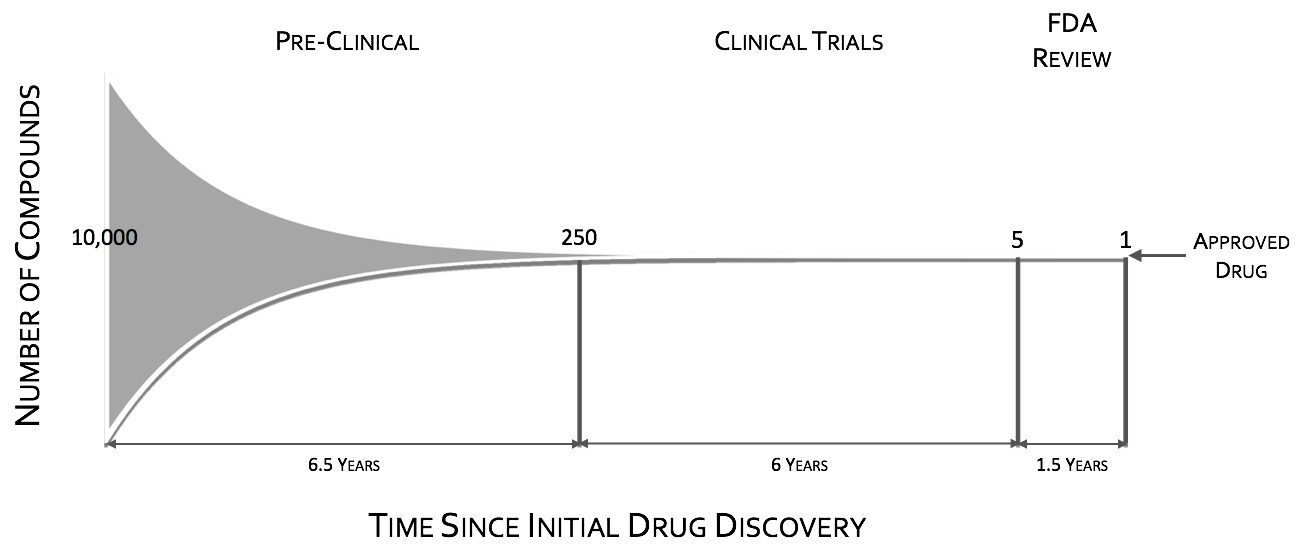

To quote the former director of National Institutes of Mental Health (NIMH), Dr. Thomas InselII, “Given that over 95% of compounds fail during the clinical phases of development (a fact not appreciated by looking at the published literature which is biased towards positive results), success may require rapid failures in order to conserve resources by moving quickly to the next candidate (“fast-fail”III). The “fast-fail, quick win” model (see figure below) is one where the proof of concept (POC) is shifted earlier in the clinical trials, from Phases II & III to Phase I, from on or after the first effective dose (FED) to the first human dose (FHD) or shortly thereafter.
Shifting the POC earlier, will increase attrition in earlier stages, but will also increase the probability of success in the new molecular entities (NME’s) that make it through phase I. This “fast-fail” approach allows a culling of the pipeline early on, reducing costV. The savings estimate due to this shift is on the order of $30 Million dollars in out-of-pocket expenses per NMEIII. This savings can be used to fund prominently 2 additional NME phase I studiesIII.
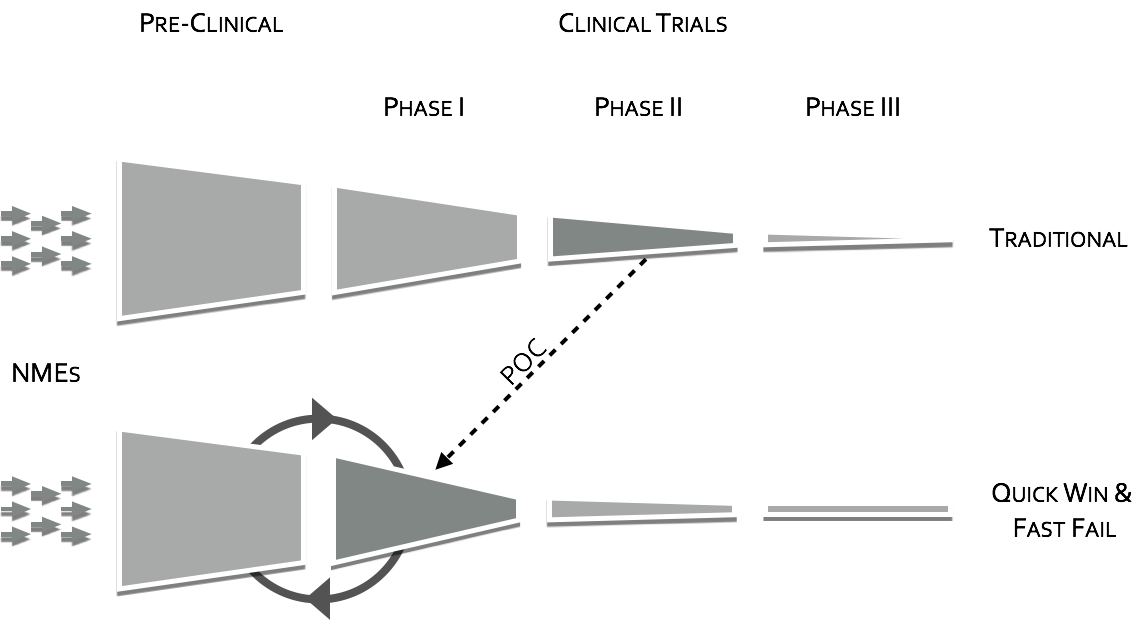
Shifting the POC to early phase will require the extra support of surrogate measures. Positron Emission Tomography (PET) has long been considered the main avenue for these surrogate makers in CNS, whether through receptor occupancy through tagged ligandsVI or through changes in glucose metabolism in the target using FDG. Unfortunately, receptor occupancy is only a part of POC, that is, it shows that the drug in question is finding the target, but it shows nothing on whether or not it is modulating the target circuit in a meaningful way. FDG has the potential to measure target modulation, but it is slow and blunt tool, and as such misses many meaningful signals. Where PET fails in measuring functional change other neuroimaging modalities can be brought to bear.
FMRI and other imaging modalities such as qEEG and EEG-fMRI are quantitative based tools, providing researchers and business leaders with statistical decision making power.